Synthesis Strategy of Pentafluorothio Group - Synthesis of Aromatic Compounds Containing SF₅

Product Manager:Nick Wilde
1. Synthetic Pathways Using SF₅Cl as the Starting Material
In 2015, the Dolbier group reported a method where phenylacetylene is used as the substrate, reacting with SF₅Cl under the catalysis of triethylborane (BEt₃) to generate a chlorinated pentafluorosulfanylated intermediate (189). Subsequent elimination of the chlorine atom with LiOH efficiently yields SF₅-substituted phenylacetylene (190). This product can further undergo 1,3-dipolar cycloaddition reactions with N-benzylaniline N-oxide (191) or benzohydroxamoyl chloride (192) to form SF₅-isoxazoline (193) and SF₅-isoxazole (194), respectively. The synthesis of isoxazole 194 requires reaction in THF for 36 hours with the addition of 5.5 equivalents of triethylamine, while the synthesis of isoxazoline 193 takes only 16 hours without additives.

In 2016, the Carreira group developed a synthetic route using vinyl acetate (51) as the substrate. In n-pentane at -40°C, SF₅Cl reacts with vinyl acetate under triethylborane (BEt₃) catalysis for 1.5 hours to form 1-chloro-2-pentafluorosulfanylethyl acetate (195). This intermediate is reduced with lithium aluminum hydride (LiAlH₄) to obtain 2-pentafluorosulfanylethanol (196), which is then oxidized with periodic acid (H₅IO₆) and chromium trioxide (CrO₃) to 2-pentafluorosulfanyl acetic acid. Finally, esterification with benzyl alcohol yields benzyl ester 197 with an overall yield of 80%. Additionally, the group successfully constructed 3-SF₅-quinolinone compounds through palladium-catalyzed hydrogenation and intramolecular condensation reactions.

In 2021, the Bizet group used N-tosyl-2-ethynylaniline (198) as the starting material to synthesize SF₅-substituted alkenes (199) in the presence of triethylborane (BEt₃) and oxygen. This intermediate can be converted to SF₅-substituted indoles through two pathways:Elimination using lithium hexamethyldisilazide (LiHMDS) in THF to obtain SF₅-substituted alkynes (200), followed by cyclization in the presence of potassium phosphate to generate 2-SF₅-substituted indole (202) and its N-Ts derivative (201).Elimination with LiOH in DMSO to directly obtain a mixture of 202 and 201.

In 2024, the Bizet group further reported a method for synthesizing 2-SF₅-indanol (205). The indanol skeleton is efficiently constructed through palladium-catalyzed coupling reaction of SF₅-substituted alkyne (204) with o-formylphenylboronic acid (203). This product can be further oxidized to 2-SF₅-indanone and used as a platform molecule in reactions such as nitration, nucleophilic addition, and Michael addition.

In 2024, the Paquin and Charette groups respectively used flow chemistry technology to synthesize SF₅-substituted pyrazole and 3H-pyrazole derivatives. Among them, SF₅-alkyne (206) reacts with diazo compound (207) at high temperature to generate regioisomers 208 and 208'; while the cycloaddition reaction of SF₅-alkene (209) with non-stabilized diazo compound (212) efficiently constructs highly substituted SF₅-3H-pyrazole (213).

2. Synthesis of SF₅ Compounds by Oxidative Fluorination
The research group of Japanese chemist Umemoto pioneered the Cl₂/KF two-component fluorination system, which forms the key Ar-SF₄Cl intermediate, followed by chlorine-fluorine exchange using ZnF₂, SbF₅, or HF to obtain the target SF₅ compound. This groundbreaking work laid an important foundation for subsequent research. In 2015, the Dolbier research team successfully applied this system to the conversion of 2,2'-bipyridine disulfide derivatives. Experiments showed that the SF₄Cl intermediate can be efficiently prepared under Umemoto's original reaction conditions in acetonitrile solvent at 0-20°C. Notably, when AgF is used as the fluorinating reagent, the pyridine-based SF₄Cl intermediate can be converted to the pentafluorosulfanylated product 216 at 60-70°C with significantly improved conversion efficiency.

The Japanese Shibata research group subsequently made important extensions to this system. They found that fluorinated bipyridine disulfide (217) first forms pyridine-SF₄Cl intermediate (218) under excess KF/Cl₂ (acetonitrile, room temperature) conditions, and after high-temperature fluorination at 120°C, meta- or para-SF₅-substituted fluoropyridines (219) are obtained in high yield. Further studies revealed that this intermediate can also undergo nucleophilic aromatic substitution (SNAr) reactions with various nucleophiles containing carbon, nitrogen, sulfur, oxygen, etc., providing new ideas for constructing diverse SF₅-pyridine derivatives.

In 2016, the Czech Beier research group reported a new method for fluorinating 1,2-bis (3-nitrophenyl) disulfide 220 using 10% F₂/N₂ mixed gas. 3-nitro-SF₅ benzene (221) was obtained in 39% yield in acetonitrile solvent at -10 to -4°C. In 2019, the team extended this method to the conversion of thiophenol( 222) and diaryl disulfide (223), with yields ranging from 8-45%. Due to the high reactivity of fluorine gas, which often leads to the formation of over-fluorinated by-products, the research team developed an innovative batch-flow hybrid process. Although 20% fluorine gas loading is required, this process significantly improves reaction selectivity and yield, providing a feasible scheme for large-scale production.

In 2017, the Shibata team achieved an important breakthrough in the conversion study of chlorotetrafluorothiophenol derivatives 227: they found that Ag₂CO₃ can efficiently prepare aryl-SF₅ compounds in dichloromethane or pure solvent (room temperature to 100°C) through a unique self-elimination Cl-F exchange mechanism without an external fluorine source; they also first confirmed that IF₅ (3-5 equivalents) can be used as an efficient fluorinating reagent to convert 227 to SF₅ derivative 228' at 65°C.

Between 2015 and 2017, the team also developed a synthetic route for SF₅-containing diaryliodonium salts (233). Starting from brominated diphenyl disulfide (229), highly reactive iodonium salts (233) were finally obtained through multiple steps of conversion. These compounds can react with various nucleophiles such as alcohols, thiols, and amines to generate structurally rich SF₅-functionalized molecules 234.

To avoid the safety risks of directly using chlorine gas, several research groups have developed alternative scheme: Pitts et al. used a trichloroisocyanuric acid (TCCA)/KF/TFA (0.1 equivalent) system to prepare Ar-SF₄Cl intermediate 236 under gas-free conditions, followed by fluorination with AgF to obtain the target product 237.

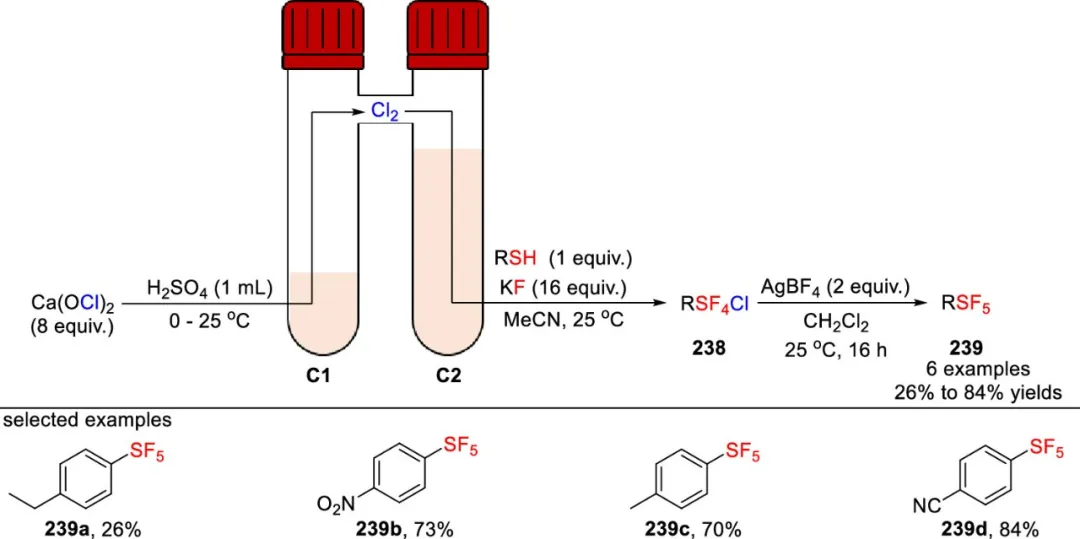
In 2023, the De Borggraeve and Ismalaj teams developed a synthetic route that does not require direct use of chlorine gas. This method uses calcium hypochlorite and sulfuric acid to in situ generate chlorine gas in a CO-ware reactor for the synthesis of chlorotetrafluorothiophenol derivatives 239 (as shown in the figure). The specific process is as follows: chlorine gas generated in the first chamber (C1) of the reactor reacts with thiol and KF in acetonitrile solvent in the second chamber (C2) at room temperature to generate chlorotetrafluorothiophenol 238; subsequently, this intermediate 238 is treated with 2 equivalents of silver tetrafluoroborate in dichloromethane to obtain the corresponding SF₅-containing compound 239.
Currently, the synthesis strategy of ArSF₅ usually adopts a two-step method: first, the ArSF₄Cl intermediate is prepared, and then it is reacted with a fluorinating reagent or silver salt to obtain ArSF₅. To simplify the synthesis process, teams such as Gatzenmeier and Nozaki have developed a direct synthesis method (Figure a), which core is a composite system composed of tetraethylammonium bromide/ammonium chloride and excess AgF (up to 16 equivalents). This system can efficiently convert disulfide or thiophenol derivatives 240 to aryl-SF₅ products 241 in acetonitrile or dichloromethane solvent at room temperature. This method can also be extended to scenarios using aryl halides/pseudohalides as raw materials, among which thiobenzoates or triphenylmethyl sulfides formed from diazonium salts have been proven to be effective precursors for the one-step synthesis of ArSF₅. Recently, the team further developed a general method for directly preparing ArSC (Ph)₃ 242 from aryl iodides/bromides under palladium catalysis, and this intermediate can be used for the one-step synthesis of ArSF₅ products 244 according to the aforementioned strategy.

In 2020, the Guzyr team reported a method for synthesizing aryl-SF₅ compounds (as shown in the figure). They first reacted disulfide 245 with chlorine gas and potassium fluoride in acetonitrile to generate ClSF₄-substituted aryl compound 246; then, it was treated with mercury oxide and HF to convert it to SF₅-substituted aryl compound 247.

Aladdin:https://www.aladdinsci.com
 首页
首页 400-620-6333
400-620-6333
