Recent Advances in the Catalytic Transformations to Access Alkylsulfonyl Fluorides as SuFEx Click Hubs
Product Manager:Nick Wilde
ABSTRACT
The quest for innovative and effective techniques to produce sulfonyl fluorides is of significant interest due to their extensive applications across various fields. Sulfur(VI) fluoride exchange (SuFEx) click chemistry has recently gained prominence as one of the leading methods in this domain. Consequently, the development of efficient catalytic methodologies for synthesizing alkylsulfonyl fluorides has become a focal point in organic chemistry. While there has been substantial progress in the synthesis of arylsulfonyl fluorides, methods for generating aliphatic sulfonyl fluorides have not been as thoroughly investigated. This review highlights recent advancements in four distinct strategies for producing alkylsulfonyl fluorides: (i) photoredox catalysis, (ii) electrocatalysis, (iii) transition-metal catalysis, and (iv) organocatalysis. These methods yield various sulfonyl fluorides that serve as bioactive compounds and building blocks for subsequent SuFEx transformations.
INTRODUCTION
Sulfonyl fluorides are vital building blocks in chemical synthesis, finding wide-ranging applications in materials science, chemical biology, and drug discovery. 1-3 Since 2014, Sharpless and his team have illustrated that the sulfur(VI) fluoride exchange(SuFEx) reaction is a promising new click chemistry approach, characterized by the unique reactivity and stability of organosulfur fluorides.4-6 The Huisgen azide-alkyne cycloaddition, recognized as the first-generation click reaction, is highly valued for its ability to ligate azides and alkynes under the influence of mild copper catalysis.7-10 Click reactions are advantageous due to their compatibility with aqueous and oxygen-tolerant environments, leading to high product yields. Compared to sulfonyl chlorides, sulfonyl fluorides exhibit greater stability under both acidic and basic conditions.11
Sulfur(VI)-containing compounds are extensively employed in the pharmaceutical industry,12-15 materials science,16 and polymer science.17 In biochemistry, sulfonyl fluorides have been used notably for protease inhibition and as biological probes (Figure 1, Part (a)).18-20 The SuFEx reaction, which occurs between di(arylsulfonyl fluorides) and di(aryl silyl ethers) to produce polysulfonate-SuFEx polymers, has shown remarkable efficiency and holds special significance in polymer science21-24. Traditional methods for synthesizing these functional molecules often require multistep processes (Figure 1, Part (b)). Efficient catalytic processes depend on well-designed and easily accessible precursors. For instance, ethenesulfonyl fluoride (ESF, H2C=CHSO2F) serves as an effective Michael acceptor for creating a variety of nitrogen-, oxygen-, and carbon-based nucleophiles, which are useful in the synthesis of functionalized alkylsulfonyl fluorides (Figure 1, Part (c)).25-27
In this comprehensive evaluation, we spotlight innovative approaches encompassing photoredox catalysis, electrocatalysis, transition-metal-mediated catalysis, and organocatalysis, all of which have emerged as powerful tools for the synthesis of alkylsulfonyl fluorides, as depicted in Figure 1, Part (d).
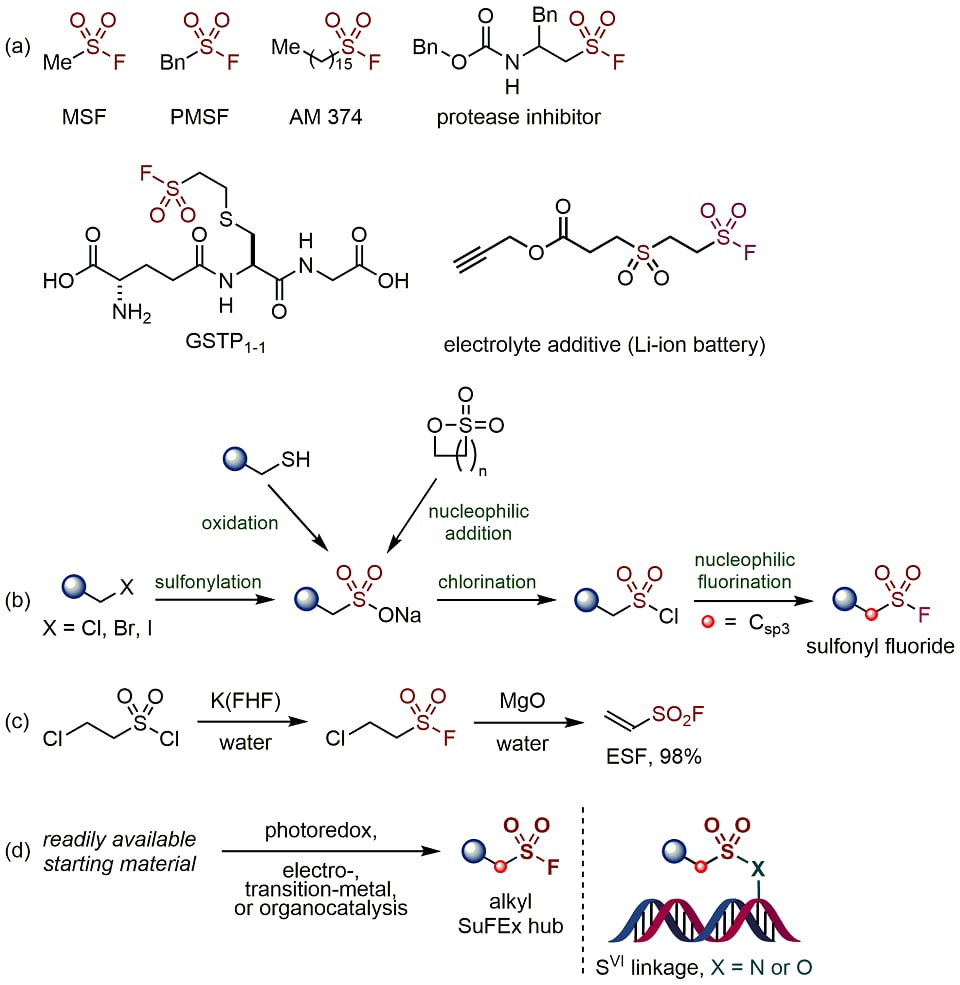
Figure 1. (a) Chemical Structures of Representative Biologically Active Alkylsulfonyl Fluorides. (b) Conventional Methods Used for the Synthesis of Alkylsulfonyl Fluorides. (c) Sharpless’s Kilogram- Scale Synthesis of ESF. (d) Catalytic Synthetic Methods Highlighted in This Review.
CONCLUSIONS AND OUTLOOK
We have delved into the synthesis of alkylsulfonyl fluorides, encompassing their formation via carbon–carbon and carbon–heteroatom bond formations, along with their fluoride exchange (SuFEx) reactions with suitable coupling agents. The manipulation of the SO2F functional group, enabled by activation techniques such as photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis, has opened up avenues to an extensive range of both carbocyclic and heterocyclic compounds. We are optimistic that these techniques will enrich the libraries of sulfonyl fluoride-containing compounds, thereby bolstering the research efforts in the fields of pharmaceuticals and agrochemicals.
Aladdin:https://www.aladdinsci.com
REFERENCE
1.Lange W, Müller E. 1930. Über Aryl‐fluorsulfonate, Ar. O. So 2 F. Ber. dtsch. Chem. Ges. A/B. 63(9):2653-2657. https://doi.org/10.1002/cber.19300630946
2.Steinkopf W. 1927. Über Aromatische Sulfofluoride. J. Prakt. Chem. 117(1):1-82. https://doi.org/10.1002/prac.19271170101
3.Steinkopf W, Jaeger P. 1930. Über Aromatische Sulfofluoride. II. Mitteilung. J. Prakt. Chem. 128(1):63-88. https://doi.org/10.1002/prac.19301280104
4.Dong J, Krasnova L, Finn MG, Sharpless KB. 2014. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem., Int. Ed. 53(36):9430-9448. https://doi.org/10.1002/anie.201309399
5.Li S, Wu P, Moses JE, Sharpless KB. 2017. Multidimensional SuFEx Click Chemistry: Sequential Sulfur(VI) Fluoride Exchange Connections of Diverse Modules Launched From An SOF4 Hub. Angew. Chem., Int. Ed. 56(11):2903-2908. https://doi.org/10.1002/anie.201611048
6.Gao B, Li S, Wu P, Moses JE, Sharpless KB. 2018. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem., Int. Ed. 57(7):1939-1943. https://doi.org/10.1002/anie.201712145
7.Huisgen R. 1963. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem., Int. Ed. 2(10):565-598. https://doi.org/10.1002/anie.196305651
8.Kolb HC, Finn MG, Sharpless KB. 2001. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed. 40(11):2004-2021. https://doi.org/10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
9.Tornøe CW, Christensen C, Meldal M. 2002. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 67(9):3057-3064. https://doi.org/10.1021/jo011148j
10.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. 2002. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 41(14):2596-2599. https://onlinelibrary.wiley.com/doi/10.1002/1521-3773(20020715)41:14%3C2596::AID-ANIE2596%3E3.0.CO;2-4
11.Suter CM. 1944. The Organic Chemistry of Sulfur: Tetracovalent Sulfur Compounds. Wiley: New York, NY. 453–572.
12.Majumdar KC, Mondal S. 2011. Recent Developments in the Synthesis of Fused Sultams. Chem. Rev. 111(12):7749-7773. https://doi.org/10.1021/cr1003776
13.Zhao C, Rakesh K, Ravidar L, Fang W, Qin H. 2019. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 162679-734. https://doi.org/10.1016/j.ejmech.2018.11.017
14.Meanwell NA. 2011. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 54(8):2529-2591. https://doi.org/10.1021/jm1013693
15.Scott KA, Njardarson JT. 2018. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. (Z). 376(1): https://doi.org/10.1007/s41061-018-0184-5
16.(a) Hou J, Lu L, Wang L, Ohma A, Ren D, Feng X, Li Y, Li Y, Ootani I, Han X, Ren W, He X, Nitta Y, Ouyang M. 2020. Thermal runaway of Lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes. Nat. Commun. 11(1):5100. https://doi.org/10.1038/s41467-020-18868-w (b) Ugata Y, Chen Y, Sasagawa S, Ueno K, Watanabe M, Mita H, Shimura J, Nagamine M, Dokko K. 2022. Eutectic Electrolytes Composed of LiN(SO2F)2 and Sulfones for Li-Ion Batteries. J. Phys. Chem. C. 126(24):10024-10034. https://doi.org/10.1021/acs.jpcc.2c02922
17.Dong J, Sharpless KB, Kwisnek L, Oakdale JS, Fokin VV. 2014. SuFEx-Based Synthesis of Polysulfates. Angew. Chem., Int. Ed. 53(36):9466-9470. https://doi.org/10.1002/anie.201403758
18.Alapafuja SO, Nikas SP, Bharathan IT, Shukla VG, Nasr ML, Bowman AL, Zvonok N, Li J, Shi X, Engen JR, et al. 2012. Sulfonyl Fluoride Inhibitors of Fatty Acid Amide Hydrolase. J. Med. Chem. 55(22):10074-10089. https://doi.org/10.1021/jm301205j
19.Shishido Y, Tomoike F, Kimura Y, Kuwata K, Yano T, Fukui K, Fujikawa H, Sekido Y, Murakami-Tonami Y, Kameda T, et al. 2017. A covalent G-site inhibitor for glutathione S-transferase Pi (GSTP1-1). Chem. Commun. 53(81):11138-11141. https://doi.org/10.1039/c7cc05829b
20.Aguilar B, Amissah F, Duverna R, S. Lamango N. 2011. Polyisoprenylation Potentiates the Inhibition of Polyisoprenylated Methylated Protein Methyl Esterase and the Cell Degenerative Effects of Sulfonyl Fluorides. CCDT. 11(6):752-762. https://doi.org/10.2174/156800911796191015
21.Gao B, Zhang L, Zheng Q, Zhou F, Klivansky LM, Lu J, Liu Y, Dong J, Wu P, Sharpless KB. 2017. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9(11):1083-1088. https://doi.org/10.1038/nchem.2796
22.Wang M, Jin H, Chen X, Lin B, Yang H. 2019. A sulfur(vi) fluoride exchange click chemistry approach towards main chain liquid crystal polymers bearing sulfate ester groups. Polym. Chem. 10(26):3657-3664. https://doi.org/10.1039/c9py00577c
23.Li S, Li G, Gao B, Pujari SP, Chen X, Kim H, Zhou F, Klivansky LM, Liu Y, Driss H, et al. 2021. SuFExable polymers with helical structures derived from thionyl tetrafluoride. Nat. Chem. 13(9):858-867. https://doi.org/10.1038/s41557-021-00726-x
24.Kim H, Zhao J, Bae J, Klivansky LM, Dailing EA, Liu Y, Cappiello JR, Sharpless KB, Wu P. 2021. Chain-Growth Sulfur(VI) Fluoride Exchange Polycondensation: Molecular Weight Control and Synthesis of Degradable Polysulfates. ACS Cent. Sci. 7(11):1919-1928. https://doi.org/10.1021/acscentsci.1c01015
25.Chen Q, Mayer P, Mayr H. 2016. Ethenesulfonyl Fluoride: The Most Perfect Michael Acceptor Ever Found?. Angew. Chem., Int. Ed. 55(41):12664-12667. https://doi.org/10.1002/anie.201601875
26.Meng Y, Wang S, Fang W, Xie Z, Leng J, Alsulami H, Qin H. 2020. Ethenesulfonyl Fluoride (ESF) and Its Derivatives in SuFEx Click Chemistry and More. Synthesis. 52(05):673-687. https://doi.org/10.1055/s-0039-1690038
27.Zheng Q, Dong J, Sharpless KB. 2016. Ethenesulfonyl Fluoride (ESF): An On-Water Procedure for the Kilogram-Scale Preparation. J. Org. Chem. 81(22):11360-11362. https://doi.org/10.1021/acs.joc.6b01423
28.Xu R, Xu T, Yang M, Cao T, Liao S. 2019. A rapid access to aliphatic sulfonyl fluorides. Nat. Commun. 10(1): https://doi.org/10.1038/s41467-019-11805-6
29.Zhang X, Fang W, Lekkala R, Tang W, Qin H. 2020. An Easy, General and Practical Method for the Construction of Alkyl Sulfonyl Fluorides. Adv. Synth. Catal. 362(16):3358-3363. https://doi.org/10.1002/adsc.202000515
30.Zhang X, Qin H. 2022. A General Procedure for the Construction of 2-Alkyl-Substituted Vinyl Sulfonyl Fluoride. Org. Lett. 24(50):9311-9315. https://doi.org/10.1021/acs.orglett.2c03936
31.Zhong T, Yi J, Chen Z, Zhuang Q, Li Y, Lu G, Weng J. 2021. Photoredox-catalyzed aminofluorosulfonylation of unactivated olefins. Chem. Sci. 12(27):9359-9365. https://doi.org/10.1039/d1sc02503a
32.Chen Z, Zhou X, Yi J, Diao H, Chen Q, Lu G, Weng J. 2022. Catalytic Decarboxylative Fluorosulfonylation Enabled by Energy-Transfer-Mediated Photocatalysis. Org. Lett. 24(13):2474-2478. https://doi.org/10.1021/acs.orglett.2c00459
33.Zhang H, Li S, Zheng H, Zhu G, Liao S, Nie X. 2022. Photocatalytic fluorosulfonylation of aliphatic carboxylic acid NHPI esters. Org. Chem. Front. 9(18):4854-4860. https://doi.org/10.1039/d2qo00861k
34.Wang P, Zhang H, Zhao M, Ji S, Lin L, Yang N, Nie X, Song J, Liao S. 2022. Radical Hydro‐Fluorosulfonylation of Unactivated Alkenes and Alkynes. Angew. Chem., Int. Ed. 61(39): https://doi.org/10.1002/anie.202207684
35.Wu X, Chu L, Qing F. 2013. Silver-Catalyzed Hydrotrifluoromethylation of Unactivated Alkenes with CF3SiMe3. Angew. Chem., Int. Ed. 52(8):2198-2202. https://doi.org/10.1002/anie.201208971
36.Nie X, Xu T, Song J, Devaraj A, Zhang B, Chen Y, Liao S. 2021. Radical Fluorosulfonylation: Accessing Alkenyl Sulfonyl Fluorides from Alkenes. Angew. Chem., Int, Ed. 60(8):3956-3960. https://doi.org/10.1002/anie.202012229
37.(a) Wang P, Zhang H, Nie X, Xu T, Liao S. 2022. Photoredox catalytic radical fluorosulfonylation of olefins enabled by a bench-stable redox-active fluorosulfonyl radical precursor. Nat. Commun. 13(1):3370. https://doi.org/10.1038/s41467-022-31089-7 (b) Zhang W, Li H, Li X, Zou Z, Huang M, Liu J, Wang X, Ni S, Pan Y, Wang Y. 2022. A practical fluorosulfonylating platform via photocatalytic imidazolium-based SO2F radical reagent. Nat. Commun. 13(1):3515. https://doi.org/10.1038/s41467-022-31296-2
38.Erchinger JE, Hoogesteger R, Laskar R, Dutta S, Hümpel C, Rana D, Daniliuc CG, Glorius F. 2023. EnT-Mediated N-S Bond Homolysis of a Bifunctional Reagent Leading to Aliphatic Sulfonyl Fluorides. J. Am. Chem. Soc. 145(4):2364-2374. https://doi.org/10.1021/jacs.2c11295
39.Francke R, Little RD. 2014. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43(8):2492. https://doi.org/10.1039/c3cs60464k
40.Meyer TH, Choi I, Tian C, Ackermann L. 2020. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis?. Chem. 6(10):2484-2496. https://doi.org/10.1016/j.chempr.2020.08.025
41.Liu J, Lu L, Wood D, Lin S. 2020. New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci. 6(8):1317-1340. https://doi.org/10.1021/acscentsci.0c00549
42.Laudadio G, Bartolomeu AdA, Verwijlen LMHM, Cao Y, de Oliveira KT, Noël T. 2019. Sulfonyl Fluoride Synthesis through Electrochemical Oxidative Coupling of Thiols and Potassium Fluoride. J. Am. Chem. Soc. 141(30):11832-11836. https://doi.org/10.1021/jacs.9b06126
43.Pupo G, Vicini AC, Ascough DMH, Ibba F, Christensen KE, Thompson AL, Brown JM, Paton RS, Gouverneur V. 2019. Hydrogen Bonding Phase-Transfer Catalysis with Potassium Fluoride: Enantioselective Synthesis of β-Fluoroamines. J. Am. Chem. Soc. 141(7):2878-2883. https://doi.org/10.1021/jacs.8b12568
44.Chen D, Nie X, Feng Q, Zhang Y, Wang Y, Wang Q, Huang L, Huang S, Liao S. 2021. Electrochemical Oxo‐Fluorosulfonylation of Alkynes under Air: Facile Access to β‐Keto Sulfonyl Fluorides. Angew. Chem., Int. Ed. 60(52):27271-27276. https://doi.org/10.1002/anie.202112118
45.Feng Q, Fu Y, Zheng Y, Liao S, Huang S. 2022. Electrochemical Synthesis of β-Keto Sulfonyl Fluorides via Radical Fluorosulfonylation of Vinyl Triflates. Org. Lett. 24(20):3702-3706. https://doi.org/10.1021/acs.orglett.2c01336
46.Zhang X, Huang Y, Qin H, Baoguo Z, Rakesh KP, Tang H. 2021. Copper-Promoted Conjugate Addition of Carboxylic Acids to Ethenesulfonyl Fluoride (ESF) for Constructing Aliphatic Sulfonyl Fluorides. ACS Omega. 6(40):25972-25981. https://doi.org/10.1021/acsomega.1c02804
47.Liu Y, Lin Q, Xiao Z, Zheng C, Guo Y, Chen Q, Liu C. 2019. Zinc‐Mediated Intermolecular Reductive Radical Fluoroalkylsulfination of Unsaturated Carbon–Carbon Bonds with Fluoroalkyl Bromides and Sulfur Dioxide. Chem.—Eur. J. 25(7):1824-1828. https://doi.org/10.1002/chem.201805526
48.Li X, Chen HJ, Wang W, Ma M, Chen Y, Li Y, Pullarkat SA, Leung P. 2019. Palladacycle promoted asymmetric hydrophosphination of α,β-unsaturated sulfonyl fluorides. J. Organomet. Chem. 899120912. https://doi.org/10.1016/j.jorganchem.2019.120912
49.Chen H, Hu Z, Qin H, Tang H. 2021. A novel three-component reaction for constructing indolizine-containing aliphatic sulfonyl fluorides. Org. Chem. Front. 8(6):1185-1189. https://doi.org/10.1039/d0qo01430c
50.Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéguez M. 2008. Enantioselective Copper-Catalyzed Conjugate Addition and Allylic Substitution Reactions. Chem. Rev. 108(8):2796-2823. https://doi.org/10.1021/cr0683515
51.Hong Y, Gandeepan P, Mannathan S, Lee W, Cheng C. 2014. Alkene-Assisted Nickel-Catalyzed Regioselective 1,4-Addition of Organoboronic Acid to Dienones: A Direct Route to All-Carbon Quaternary Centers. Org. Lett. 16(11):2806-2809. https://doi.org/10.1021/ol500838h
52.Moku B, Fang W, Leng J, Kantchev EAB, Qin H. 2019. Rh(I)–Diene-Catalyzed Addition of (Hetero)aryl Functionality to 1,3-Dienylsulfonyl Fluorides Achieving Exclusive Regioselectivity and High Enantioselectivity: Generality and Mechanism. ACS Catal. 9(11):10477-10488. https://doi.org/10.1021/acscatal.9b03640
53.Sidera M, Fletcher SP. 2015. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem. 7(11):935-939. https://doi.org/10.1038/nchem.2360
54.Takaya Y, Ogasawara M, Hayashi T, Sakai M, Miyaura N. 1998. Rhodium-Catalyzed Asymmetric 1,4-Addition of Aryl- and Alkenylboronic Acids to Enones. J. Am. Chem. Soc. 120(22):5579-5580. https://doi.org/10.1021/ja980666h
55.Qin H, Zheng Q, Bare GAL, Wu P, Sharpless KB. 2016. A Heck–Matsuda Process for the Synthesis of β‐Arylethenesulfonyl Fluorides: Selectively Addressable Bis‐electrophiles for SuFEx Click Chemistry. Angew. Chem., Int. Ed. 55(45):14155-14158. https://doi.org/10.1002/anie.201608807
56.Barrow AS, Smedley CJ, Zheng Q, Li S, Dong J, Moses JE. 2019. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48(17):4731-4758. https://doi.org/10.1039/c8cs00960k
57.Moku B, Fang W, Leng J, Li L, Zha G, Rakesh K, Qin H. 2019. Rh-Catalyzed Highly Enantioselective Synthesis of Aliphatic Sulfonyl Fluorides. iScience. 21695-705. https://doi.org/10.1016/j.isci.2019.10.051
58.Colby DA, Bergman RG, Ellman JA. 2010. Rhodium-Catalyzed C−C Bond Formation via Heteroatom-Directed C−H Bond Activation. Chem. Rev. 110(2):624-655. https://doi.org/10.1021/cr900005n
59.Deb A, Bag S, Kancherla R, Maiti D. 2014. Palladium-Catalyzed Aryl C–H Olefination with Unactivated, Aliphatic Alkenes. J. Am. Chem. Soc. 136(39):13602-13605. https://doi.org/10.1021/ja5082734
60.De Almeida MV, Barton DHR, Bytheway I, Ferreira JA, Hall MB, Liu W, Taylor DK, Thomson L. 1995. Preparation and Thermal Decomposition of N,N'-Diacyl-N,N'-Dialkoxyhydrazines: Synthetic Applications and Mechanistic Insights. J. Am. Chem. Soc. 117(17):4870-4874. https://doi.org/10.1021/ja00122a018
61.Fabry DC, Zoller J, Raja S, Rueping M. 2014. Combining Rhodium and Photoredox Catalysis for CH Functionalizations of Arenes: Oxidative Heck Reactions with Visible Light. Angew. Chem., Int. Ed. 53(38):10228-10231. https://doi.org/10.1002/anie.201400560
62.Wang S, Li C, Leng J, Bukhari SNA, Qin H. 2018. Rhodium(iii)-catalyzed Oxidative Coupling of N-Methoxybenzamides and Ethenesulfonyl fluoride: a C–H Bond Activation Strategy for the Preparation of 2-Aryl ethenesulfonyl fluorides and Sulfonyl fluoride Substituted γ-Lactams. Org. Chem. Front. 5(9):1411-1415. https://doi.org/10.1039/c7qo01128h
63.Yi J, Zhou X, Chen Q, Chen Z, Lu G, Weng J. 2022. Copper-catalyzed direct decarboxylative fluorosulfonylation of aliphatic carboxylic acids. Chem. Commun. 58(67):9409-9412. https://doi.org/10.1039/d2cc03221j
64.Li Y, Chang X, Xiong Q, Dong X, Wang C. 2021. Cu-catalyzed endo-selective asymmetric 1,3-dipolar cycloaddition of azomethine ylides with ethenesulfonyl fluorides: Efficient access to chiral pyrrolidine-3-sulfonyl fluorides. Chin. Chem. Lett. 32(12):4029-4032. https://doi.org/10.1016/j.cclet.2021.05.063
65.Chen J, Zhang Y, Zhu D, Zhang X, Yan M. 2022. Construction of Chiral Quaternary Carbon Stereocenters by Asymmetric Michael Addition of 4‐Amido‐5‐hydroxylpyrazoles to Ethylene Sulfonyl Fluoride. Asian J. Org. Chem. 11(4): https://doi.org/10.1002/ajoc.202200063
66.Chen J, Zhu D, Zhang X, Yan M. 2021. Highly Enantioselective Addition of N-2,2,2-Trifluoroethylisatin Ketimines to Ethylene Sulfonyl Fluoride. J. Org. Chem. 86(3):3041-3048. https://doi.org/10.1021/acs.joc.0c02511
67.Zhu D, Zhang X, Yan M. 2021. Enantioselective Addition of Azlactones to Ethylene Sulfonyl Fluoride via Dual Catalysis. Org. Lett. 23(11):4228-4232. https://doi.org/10.1021/acs.orglett.1c01193
68.Chen J, Huang B, Wang Z, Zhang X, Yan M. 2019. Asymmetric Conjugate Addition of Ethylene Sulfonyl Fluorides to 3-Amido-2-oxindoles: Synthesis of Chiral Spirocyclic Oxindole Sultams. Org. Lett. 21(23):9742-9746. https://doi.org/10.1021/acs.orglett.9b03911
69.Lee SB, Park JH, Bae HY. 2022. Hydrophobic Amplification Enabled High‐Turnover Phosphazene Superbase Catalysis. ChemSusChem. 15(15): https://doi.org/10.1002/cssc.202200634
70.Grdadolnik J, Merzel F, Avbelj F. 2017. Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proc. Natl. Acad. Sci. U.S.A. 114(2):322-327. https://doi.org/10.1073/pnas.1612480114
71.Delany EG, Fagan C, Gundala S, Zeitler K, Connon SJ. 2013. Aerobic oxidation of NHC-catalysed aldehyde esterifications with alcohols: benzoin, not the Breslow intermediate, undergoes oxidation. Chem. Commun. 49(58):6513. https://doi.org/10.1039/c3cc42597e
72.Park JH, Lee SB, Koo BJ, Bae HY. 2022. β‐Aminosulfonyl Fluorides via Water‐Accelerated N‐Heterocyclic Carbene Catalysis. ChemSusChem. 15(18): https://doi.org/10.1002/cssc.202201000
73.Bae HY, Song CE. 2015. Unprecedented Hydrophobic Amplification in Noncovalent Organocatalysis “on Water”: Hydrophobic Chiral Squaramide Catalyzed Michael Addition of Malonates to Nitroalkenes. ACS Catal. 5(6):3613-3619. https://doi.org/10.1021/acscatal.5b00685
74.Pirrung MC. 2006. Acceleration of Organic Reactions through Aqueous Solvent Effects. Chem.—Eur. J. 12(5):1312-1317. https://doi.org/10.1002/chem.200500959
75.Park JH, González-Montiel GA, Cheong PH, Bae HY. 2023. Alkyl Sulfonyl Fluorides Incorporating Geminal Dithioesters as SuFEx Click Hubs via Water-Accelerated Organosuperbase Catalysis. Org. Lett. 25(7):1056-1060. https://doi.org/10.1021/acs.orglett.2c04224
76.Frye NL, Daniliuc CG, Studer A. 2022. Radical 1‐Fluorosulfonyl‐2‐alkynylation of Unactivated Alkenes. Angew. Chem., Int. Ed. 61(12): https://doi.org/10.1002/anie.202115593
77.Shavnya A, Coffey SB, Hesp KD, Ross SC, Tsai AS. 2016. Reaction of Alkyl Halides with Rongalite: One-Pot and Telescoped Syntheses of Aliphatic Sulfonamides, Sulfonyl Fluorides, and Unsymmetrical Sulfones. Org. Lett. 18(22):5848-5851. https://doi.org/10.1021/acs.orglett.6b02894
78.Liu Y, Wu H, Guo Y, Xiao J, Chen Q, Liu C. 2017. Trifluoromethylfluorosulfonylation of Unactivated Alkenes Using Readily Available Ag(O2CCF2SO2F) and N‐Fluorobenzenesulfonimide. Angew. Chem., Int. Ed. 56(48):15432-15435. https://doi.org/10.1002/anie.201709663
79.Lin Q, Liu Y, Xiao Z, Zheng L, Zhou X, Guo Y, Chen Q, Zheng C, Liu C. 2019. Intermolecular oxidative radical fluoroalkylfluorosulfonylation of unactivated alkenes with (fluoroalkyl)trimethylsilane, silver fluoride, sulfur dioxide and N-fluorobenzenesulfonimide. Org. Chem. Front. 6(4):447-450. https://doi.org/10.1039/c8qo01192c
80.Ma Z, Liu Y, Ma X, Hu X, Guo Y, Chen Q, Liu C. 2022. Aliphatic sulfonyl fluoride synthesis via reductive decarboxylative fluorosulfonylation of aliphatic carboxylic acid NHPI esters. Org. Chem. Front. 9(4):1115-1120. https://doi.org/10.1039/d1qo01655e
81.Tang L, Yang Y, Wen L, Yang X, Wang Z. 2016. Catalyst-free radical fluorination of sulfonyl hydrazides in water. Green Chem. 18(5):1224-1228. https://doi.org/10.1039/c5gc02755a
82.Talko A, Barbasiewicz M. 2018. Nucleophilic Fluorination with Aqueous Bifluoride Solution: Effect of the Phase-Transfer Catalyst. ACS Sustainable Chem. Eng. 6(5):6693-6701. https://doi.org/10.1021/acssuschemeng.8b00489
83.Rojas JJ, Croft RA, Sterling AJ, Briggs EL, Antermite D, Schmitt DC, Blagojevic L, Haycock P, White AJP, Duarte F, et al. 2022. Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides. Nat. Chem. 14(2):160-169. https://doi.org/10.1038/s41557-021-00856-2
84.Croft RA, Mousseau JJ, Choi C, Bull JA. 2018. Lithium‐Catalyzed Thiol Alkylation with Tertiary and Secondary Alcohols: Synthesis of 3‐Sulfanyl‐Oxetanes as Bioisosteres. Chem.—Eur. J. 24(4):818-821. https://doi.org/10.1002/chem.201705576
 首页
首页 400-620-6333
400-620-6333
