计算溶液所需的质量、体积或浓度。
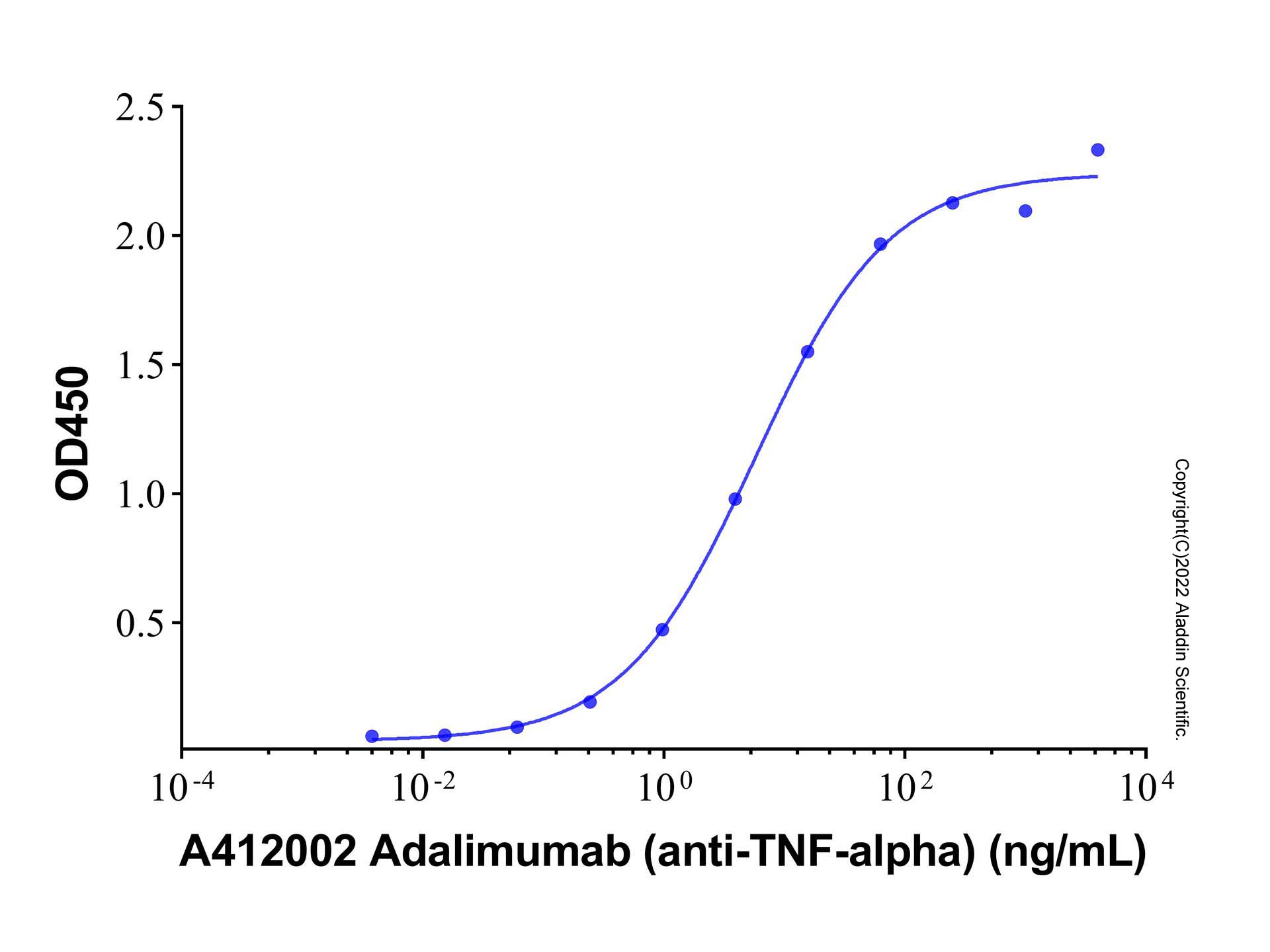
Adalimumab (anti-TNF-alpha) (A412002) - ELISA
Immobilized Recombinant Human TNF-α Protein (rp156007) at 1.0 μg/mL can bind Golimumab (anti-TNFa) (Ab175552) with the EC₅₀ of 5.78 ng/mL.

| 货号 (SKU) | 包装规格 | 是否现货 | 价格 | 数量 |
|---|---|---|---|---|
| A412002-100μg |
100μg |
现货  |
| |
| A412002-1mg |
1mg |
现货  |
| |
| A412002-5mg |
5mg |
现货  |
| |
| A412002-10mg |
10mg |
期货  |
|
| 产品名称 | Adalimumab (anti-TNF-alpha), TNF-α 抑制剂 |
|---|---|
| 别名 | 阿达木单抗(抗 TNF-alpha) |
| 英文别名 | dl-N-Acetylhomocysteinethiolactone | ONO5046;LY544349;EI546 | SIVELESTAT [INN] | SIVELESTAT [MART.] | 2,2-Dimethyl-propionic acid 4-[2-(carboxymethyl-carbamoyl)-phenylsulfamoyl]-phenyl ester | P,.ALPHA.,.ALPHA.-TRIMETHYLBENZYL ALCOHOL [FHFI] | J-005486 | |
| 规格或纯度 | 无载体, 重组, ExactAb™, 无叠氮钠, 已验证, 无动物源, ≥95%(SDS-PAGE&SEC-HPLC), 见COA |
| 宿主种属 | 人(Human) |
| 特异性 | TNFSF2/TNFa |
| 应用 | ELISA,Functional Assay,Flow cytometry,Kinetics (BLI),Kinetics (SPR) |
| 种属反应性 | 人(Human),小鼠(Mouse),恒河猴(Rhesus monkey),食蟹猴(Cynomolgus monkey) |
| 偶联 | Unconjugated |
| 作用类型 | 抑制剂 |
| 作用机制 | TNF-α 抑制剂 |
| 抗体类型 | Primary antibody |
|---|---|
| 克隆类型 | 重组抗体 |
| Format | Whole IgG |
| 亚型 | Human IgG1 |
| 轻链亚型 | kappa |
| SDS-PAGE | 145.5 kDa |
| 纯化方法 | Protein A purified |
| 纯度 | >95% (SDS-PAGE&SEC) |
| 来源 | CHO supernatant |
| 物理外观 | Liquid |
| 储存缓冲液 | Supplied as a 0.22 μm filtered solution in 100mM Pro-Ac, 20mM Arg, pH 5.0 |
| 防腐剂 | No |
| 浓度 | 见COA |
| 储存温度 | -80℃储存,避免反复冻融 |
| 运输条件 | 超低温冰袋运输 |
| 稳定性与储存 | 在 -80℃ 下保存 24 个月。收货后建议分装。避免冷冻/解冻循环。 |
| CAS编号和信息 | 331731-18-1 |
| 分子类型 | 抗体 |

Adalimumab (anti-TNF-alpha) (A412002) - ELISA
Immobilized Recombinant Human TNF-α Protein (rp156007) at 1.0 μg/mL can bind Golimumab (anti-TNFa) (Ab175552) with the EC₅₀ of 5.78 ng/mL.
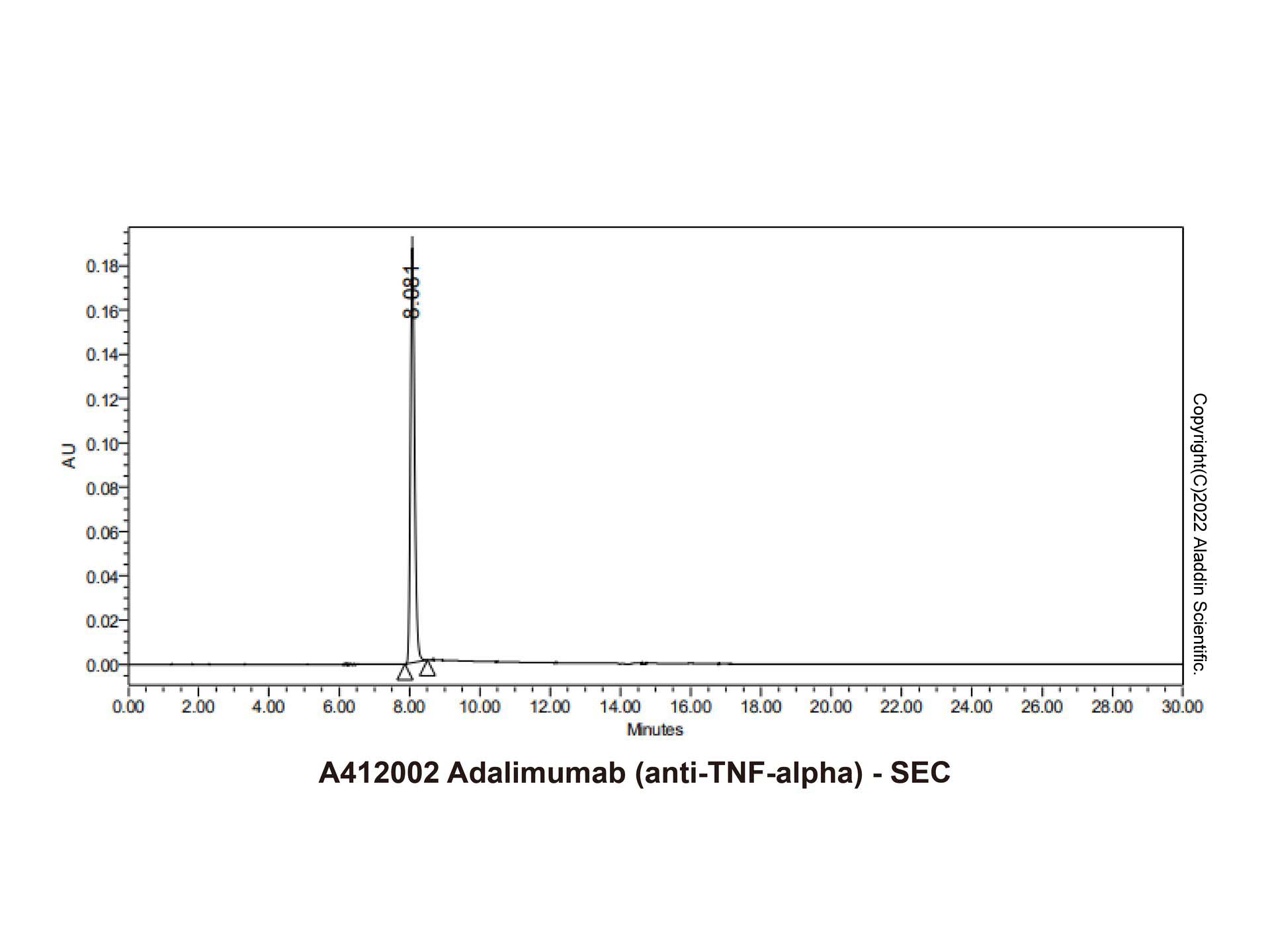
Adalimumab (anti-TNF-alpha) (A412002) - SEC
The purity of Adalimumab (anti-TNF-alpha) (A412002) is more than 95% verified by HPLC.
| 作用机制 | Action Type | target ID | Target Name | Target Type | Target Organism | Binding Site Name | 参考文献 |
|---|
通过匹配包装上的批号来查找并下载产品的 COA,每批产品都进行了严格的验证,您可放心使用!
| 批号(Lot Number) | 证书类型 | 日期 | 货号 |
|---|---|---|---|
| 分析证书 | 24-03-26 | A412002 | |
| 分析证书 | 24-03-26 | A412002 | |
| 分析证书 | 24-03-26 | A412002 |

¥669.90

¥379.90

¥199.90

¥669.90

¥379.90

¥639.90

¥669.90

¥419.90
| 1. Kempeni J. (1999) Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7.. Ann Rheum Dis, 58 Suppl 1 (I70-2). [PMID:10577977] |
| 2. Wollheim FA. (2002) TNF inhibition as therapy for rheumatoid arthritis.. Expert Opin Investig Drugs, 11 (7): (947-53). [PMID:12084005] |
| 3. Rau R. (2002) Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials.. Ann Rheum Dis, 61 Suppl 2 (ii70-3). [PMID:12379628] |
| 4. Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, Barisani-Asenbauer T, Franco P, Heiligenhaus A, Scales D et al.. (2016) Adalimumab in Patients with Active Noninfectious Uveitis.. N Engl J Med, 375 (10): (932-43). [PMID:27602665] |
| 5. Kawalec P, Mikrut A, Wiśniewska N, Pilc A. (2013) Tumor necrosis factor-α antibodies (infliximab, adalimumab and certolizumab) in Crohn's disease: systematic review and meta-analysis.. Arch Med Sci, 9 (5): (765-779). [PMID:24273556] |
| 6. Puri A, Niewiarowski A, Arai Y, Nomura H, Baird M, Dalrymple I, Warrington S, Boyce M. (2017) Pharmacokinetics, safety, tolerability and immunogenicity of FKB327, a new biosimilar medicine of adalimumab/Humira, in healthy subjects.. Br J Clin Pharmacol, 83 (7): (1405-1415). [PMID:28133772] |
| 7. Jani RH, Gupta R, Bhatia G, Rathi G, Ashok Kumar P, Sharma R, Kumar U, Gauri LA, Jadhav P, Bartakke G et al.. (2016) A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis.. Int J Rheum Dis, 19 (11): (1157-1168). [PMID:26176644] |
| 8. Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, Philipp S, Spelman L, Zhang N, Strober B. (2017) Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis.. Br J Dermatol, 177 (6): (1562-1574). [PMID:28755394] |
| 9. Kronthaler U, Fritsch C, Hainzl O, Seidl A, da Silva A. (2018) Comparative functional and pharmacological characterization of Sandoz proposed biosimilar adalimumab (GP2017): rationale for extrapolation across indications.. Expert Opin Biol Ther, 18 (8): (921-930). [PMID:29962245] |
| 10. Schreiber S, Yamamoto K, Muniz R, Iwura T. (2020) Physicochemical analysis and biological characterization of FKB327 as a biosimilar to adalimumab.. Pharmacol Res Perspect, 8 (3): (e00604). [PMID:32500668] |